Abstract
Introduction: Although spinal anesthesia (SA) may reduce postoperative morbidity, most urologists perform flexible ureterorenoscopy (fURS) under general anesthesia (GA). The objective of our study is to provide technical details, results, complications, and patients’ satisfaction with fURS performed under exclusive SA. Methods: We analyzed all consecutive fURS performed under SA to treat renal stones from March 2011 to February 2017. Details of technique, operative time, outcomes, need for further treatments, complications, and patients’ satisfaction were evaluated. Results: Two hundred thirty-four fURS under SA were considered. SA was performed through L2-L3 vertebral interspace in 64.1%. Patients were discharged the same day of surgery. Mean stone burden was 13.5 ± 6.6 mm and mean operative time 76.9 ± 34.6 min. Single-procedure SFR was 69.7%. Further treatments were performed in 22.8%. 96.6% had no anesthesia-related complications. No Clavien-Dindo grade ≥ IIIb complications were noticed. 99.6% of patients were satisfied with SA. No cases of conversion from SA to GA occurred. Conclusion: fURS can be performed safely and efficiently under SA, taking into account a few details of the technique. Patients’ satisfaction with SA is very high, and complications are rare. Although SA is usually adopted in unfit patients for GA, it may be considered as a viable alternative in fit patients.
Introduction
Flexible ureterorenoscopy (fURS) has increasingly become a common treatment for kidney stones [1‒3]. Although it has been demonstrated that spinal anesthesia (SA) may reduce postoperative morbidity and mortality compared to general anesthesia (GA) [4], most urologists choose to perform fURS under GA [5], according to experts’ opinions and personal preference [1, 2]. Very limited data are available concerning fURS under SA [6, 7]. In our department, fURS has been commonly performed under SA since 2008, in order to reduce GA-related postoperative morbidity. Similar stone-free and complication rates between fURS under SA and GA were previously reported by our group [6]. The objective of our work was to share the results and complications of our large series of fURS under SA, focusing on anesthesiological and surgical details of technique and patients’ satisfaction.
Materials and Methods
A prospectively maintained database was analyzed. All consecutive fURS under SA for kidney stone treatment from March 2011 to February 2017 were included. The procedures were performed at the Department of Urology of Città della Salute e della Scienza – Molinette University Hospital (Turin, Italy). Patients’ (sex, age, American Society of Anesthesiology – ASA score, and Charlson’s Comorbidity Index – CCI) and stones’ (location, size, number, and total stone burden) characteristics were evaluated. Before fURS, patients underwent abdominal ultrasound (US) and kidney-ureter-bladder (KUB) X-ray performed by independent radiologists. The largest diameter was used to assess stone size.
Spinal Anesthesia
With a seated patient, after sterile preparation, local anesthetic at the intended spinous interspace was administered. SA was performed using a Sprotte needle between L1-L2 and L4-L5 vertebral interspace, administering bupivacaine alone or in association with fentanyl. SA was performed by a member of the Anesthesiology Staff of the Urology Day Surgery of Città della Salute e della Scienza – Molinette University Hospital. The following characteristics were recorded concerning spinal anesthesia: vertebral interspace where lumbar puncture was performed, size (gauge) of the Sprotte needle, drug and dose of local anesthetic, patients’ satisfaction with anesthesia, and postoperative pain (VAS scale) assessed through a specific questionnaire at discharge and at postoperative consultation.
Surgery
First, semirigid ureteroscopy was performed. A 10.7/12.7-Fr or 11/13-Fr ureteral access sheath (UAS) was inserted before fURS, performed with Flex-X2 (Karl Storz, Tuttlingen, DE) or Viper TM (Richard Wolf, Vernon Hills, USA) fiberoptic flexible ureterorenoscopes. A continuous irrigation with saline was used, maintaining a <40-cm water (30 mm Hg) pressure by checking the continuous outflow from the UAS, in order to avoid high intrarenal pressure. Thirty-Watt holmium laser was used for lithotripsy. The dusting technique was usually preferred, while significant residual fragments were then removed with a nitinol basket. fURS was performed by a member of the Endourology Staff of the Urology Department of Città della Salute e della Scienza – Molinette University Hospital. The following surgical technique characteristics were considered: antibiotic prophylaxis administration, semirigid ureteroscopy performed before fURS, access sheath use, type of irrigation system, type of energy and technique for lithotripsy, use of retrograde pyelography, double J stenting, and bladder catheter placement and removal.
Outcome Measures
Single-procedure outcomes were evaluated. The rate of conversion from SA to GA, stone-free rate (SFR), need for further treatments, operative time, and complication rate were analyzed. SFRs were evaluated through US and KUB X-ray performed by independent radiologists 3–4 weeks after fURS. SFRs were defined according to SFR levels proposed by Somani et al. [8]. Patients were considered SF in case both US and KUB X-ray did not show any residual fragments larger than 4 mm (SFR-4UX) [8, 9]. Surgical complications were recorded at discharge and during following urological consultations and were classified according to Clavien-Dindo classification [10]. The study was performed according to the principles of the Declaration of Helsinki. A written informed consent was obtained from all individual participants included in the study. According to the Italian law, an institutional review board approval was waived due to the prospective observational nature of this research.
Statistical Analysis
Descriptive statistical analysis was performed. All continuous variables were normally distributed and presented as mean ± standard deviation, while categorical variables were presented as fractions.
Results
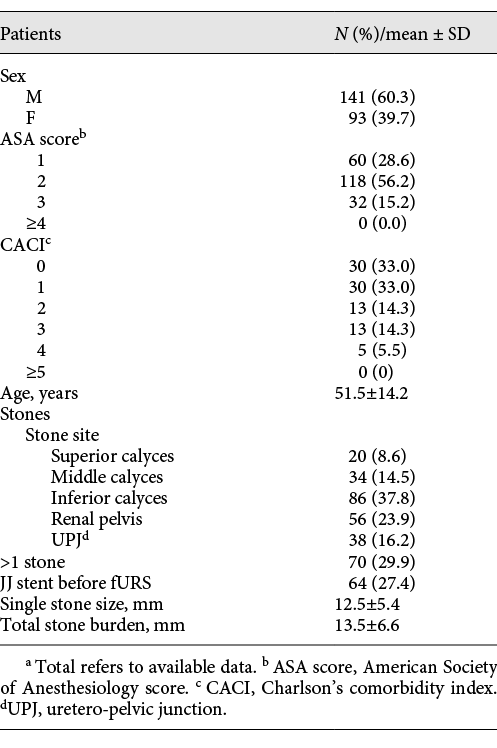
Two hundred thirty-four fURS under SA were considered. The majority of patients were male (60.3%) and ASA score 2 (56.2%) and CCI score 0–1 (65.9%). Mean age was 51.5 ± 14.2 years. Mean single stone size was 12.5 ± 5.4 mm. Mean total stone burden was 13.5 ± 6.6 mm. Multiple stones were treated in 29.9%. 27.4% of patients had an indwelling double J stent placed before fURS. Patients’ and stones’ characteristics are reported in detail in Table 1.
Spinal Anesthesia
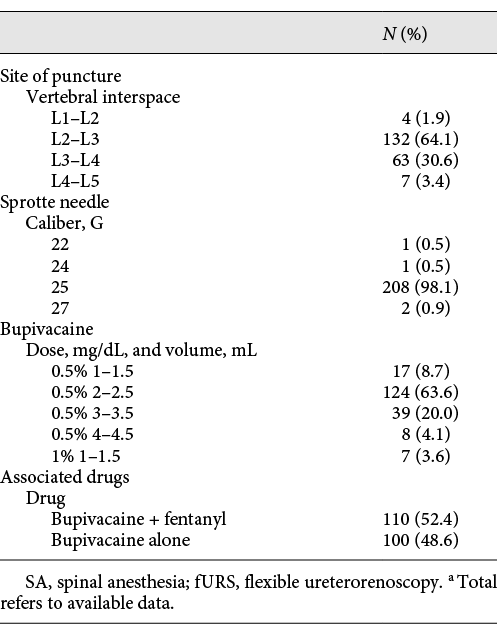
Patients were apprised of the benefits and risks related to SA. The spine of L4 was located as crossed by Tuffier’s line (joining iliac crests). Lumbar puncture was performed in the L2/L3 (64.1%) or L3/L4 (30.6%) vertebral interspace through a 25-gauge (G) Sprotte needle in the vast majority of cases (98.1%). Hyperbaric bupivacaine 0.5% 1.5–4 mL was most commonly used, often combined with fentanyl (52.4%). More details about SA are shown in Table 2.
Surgery
All the endoscopic procedures were performed under antibiotic prophylaxis. Semirigid ureteroscopy was always performed. A UAS 10.7/12.7 Fr or 11/13 Fr was always inserted before fURS. A retrograde pyelography was always performed, before placing the double J stent and a Foley catheter at the end of the procedure. The urinary catheter was removed, and patients were discharged the same day of surgery, after assessing adequate pain control and spontaneous micturition.
Outcome Measures
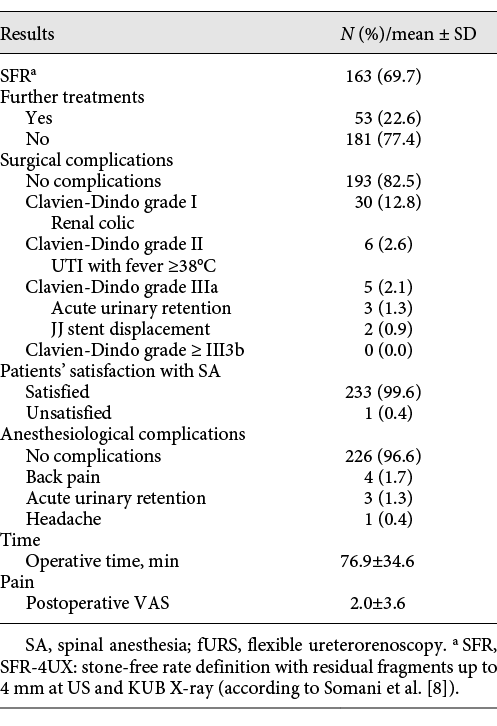
Single-procedure SFR was 69.7%. Further treatments (fURS or ESWL) were performed in 22.6% of cases. Mean operative time was 76.9 ± 34.6 min. 96.6% had no anesthesia-related complications. 1.7% of patients complained of back pain after SA, 1.3% had an episode of acute urinary retention after catheter removal, and 0.4% suffered from a headache after SA. Mean postoperative VAS for pain was 2.0 ± 3.6. Clavien-Dindo grade I complications (pain requiring analgesic drugs) occurred in 12.8% of patients, grade II (UTI with fever ≥38°C treated with antibiotics) in 2.6%, and grade IIIa in 2.1% (3 acute urinary retention and 2 JJ stent displacement). No grade IIIb, IV, and V complications occurred. 99.6% of patients were satisfied with SA. No case of conversion from SA to GA occurred. Table 3 reports in detail the outcomes and complications from our series of fURS under SA.
Discussion
fURS is commonly performed under GA, according to leading experts’ advice [1] and widespread practice [5]. Very limited data are available focusing on fURS under exclusive SA. Zeng et al. [11] wrote about fURS under combined epidural-SA in 31 patients with a mean stone size of 1.9 ± 0.9 mm; SA was associated with epidural anesthesia, provided through the epidural catheter placed in thoracic intervertebral spaces. Moreover, the mean stone size was quite small, so results should be cautiously interpreted.
In our department, fURS has been commonly performed solely under SA since 2008, in order to reduce GA-related postoperative morbidity. Our series of 234 fURS is the first large one in the literature performed under exclusive SA (not combined with epidural anesthesia). Our results show that fURS is feasible, safe, and effective under SA, patients’ satisfaction with SA is very high, and complications are rare.
SA has been shown to have advantages compared to GA: GA is linked to an increased risk of anaphylaxis, pulmonary, vascular, and neurologic complications, due to the administration of multiple drugs and potential injuries related to the endotracheal tube [12]. Furthermore, while advanced age is a well-known risk factor for anesthesia complications, regional anesthesia was shown to reach better outcomes than GA in elderly patients, in terms of mortality, delirium, intensive care unit admission, and ventilator care [13]. Both in PCNL [14, 15] and in semirigid ureteroscopy [16], SA showed better postoperative pain control, higher patient satisfaction, and shorter hospital stay compared to GA, hence reducing costs. Moreover, the literature failed to notice a higher incidence of ureteral complications in semirigid ureteroscopies performed under SA [5].
Nevertheless, leading experts recommend to perform fURS under GA [1, 2], and even if few unpublished personal experience exists, data on fURS under exclusive SA are totally lacking in the literature, in particular about complications and patient satisfaction. SA can also provide some other advantages: patients may watch the screen and be aware of the accuracy and difficulty of the operation. This is particularly important when, for anatomical or time reasons, it is not possible to achieve a stone-free status at the end of a single procedure. On the other hand, in case of anxious patients, longer procedures, or whenever requested by patients, anesthesiologists can promptly add some sedatives (such as i.v. midazolam), and patients can sleep. In our study, patient satisfaction with SA was very high, and procedures were all completed under SA with no need of conversion to GA, confirming that they were very well tolerated by patients.
It has to be pointed out that SA has also some disadvantages compared to GA, and some surgical details could help minimize drawbacks and optimize fURS outcomes. The management of breathing movements during lithotripsy is one of the main reasons why some experts prefer GA for fURS [1, 2]. SA may be less comfortable for surgeons, especially during laser lithotripsy: this is particularly true in patients with a prevalent abdominal breathing and in longer procedures. However, the extent of respiratory acts can be decreased just asking the patient to reduce it or even to stop breathing for a while during the most critical phases of laser lithotripsy. The chance of stopping mechanical ventilation has also been advocated as one of the advantages of GA [1, 2]. Induced apnea technique was described to increase lithotripsy efficiency [17], but potential hypercapnea could be a concern. SA allows patients themselves to control the length of apnea periods, thus reducing the risk of hypercapnea. In addition, experienced surgeons can predict and adapt to respiratory excursion: sometimes, breathing movements can be even used to achieve valid lithotripsy, staying still with the scope and letting the stone move in front of it [18]. On the other hand, patients are asked, at the beginning of the procedure, to promptly warn the surgeon in case they feel the need of moving, coughing, or complaining of pain during SA: thus, the surgeon can wait still, while the patient is assisted by the anesthesiologist. Moreover, a mild sedation might be added to SA in the rare case if the patient feels uncomfortable or upon patient’s request.
Another advocated reason to prefer GA is that, especially for larger stones, the SA time frame might be exceeded, requiring subsequent general anesthesia to complete the procedure or further fURS to improve the outcome. In this regard, it should be pointed out that the duration of a proper SA can exceed 2 h depending on the combination of drugs and dose [19], while the suggested duration of fURS should not exceed a couple of hours for safety purposes [1, 8, 20‒23]. However, in case of large stone burden, fURS under GA, multiple-step procedures, or percutaneous access might be considered [24].
Since some time ago, we have also altered the way work due to the current COVID-19 pandemic. In this context, SA can also allow to spare anesthesia machines and ventilators and avoid potentially risky procedures such as intubation, minimizing aerosolization [25, 26]. One shortcoming of our research is that noncontrast-enhanced computed tomography was not standardly used to evaluate stones and outcomes. Consequently, it was not possible to assess stone density, as well as stone composition. However, outcomes were evaluated with the combination of US and KUB X-ray that represent a common standard evaluation method in clinical practice in many institutions [1]. Some may wonder if the outcomes were compromised by unpredictable respiratory motion under SA, but our group has already compared the outcomes of fURS under SA with those of fURS performed under GA in a previous study, showing that SFR and complications were comparable [6].
The high sensibility of US performed by independent radiologists and the risk of US overestimation of true stone size [27] could have negatively influenced our results. The relatively low SFR of our study could also be partially explained by our choice to analyze single-procedure outcomes at a relatively short time – 3–4 weeks – after fURS.
In conclusion, our series of fURS under SA is the largest published in the literature so far and allows us to show that fURS under SA is feasible, safe, and effective, when some anesthesiological and surgical details of technique are taken into account. We also demonstrated for the first time that patients’ satisfaction with SA is very high, and complications are rare. Hence, SA may be considered not only in patients unfit for GA but also as a viable alternative in fit patients. Surgical and anesthesiological details in this study can help endourologists to minimize SA drawbacks and optimize fURS outcomes and patients’ satisfaction.
Statement of Ethics
The study was conducted ethically in accordance with the principles of the World Medical Association Declaration of Helsinki. A written informed consent was obtained from all individual participants included in the study. According to the Italian law, an institutional review board approval was waived due to the prospective observational nature of this research.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
The authors did not receive any funding.
Author Contributions
The authors listed have made substantial contributions to the intellectual content of the study in the various sections described: A. Bosio contributed to conception and design, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, and statistical analysis; E. Alessandria contributed to drafting of the manuscript and critical revision of the manuscript for important intellectual content; F. Vitiello, E. Vercelli, and S. Agosti contributed to acquisition of data; P. Gontero contributed to supervision.
Data Availability Statement
Data are available for bona fide researchers on request from the authors.