Abstract
Oligometastatic prostate cancer (PCa) can be defined as cancer with a limited number of metastases, typically fewer than 5 lesions, and involves lesions contained within the axial versus the appendicular skeleton. Patients can present with de novo oligometastatic, oligorecurrent, or oligoprogressive PCa. Oligometastatic PCa patients demonstrate considerable improvements in survival outcomes, with a better prognosis than patients with extensive metastatic disease. However, the management of patients that present with nonsymptomatic oligometastatic PCa remains difficult. In the oligometastatic setting, the benefit of local therapies such as prostatectomy and radiotherapy on survival outcomes is an intriguing topic; however, their impact on oncological outcomes is still unknown.
Introduction
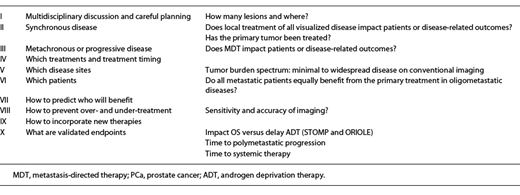
Prostate cancer (PCa) is the most commonly diagnosed life-threatening cancer in men (164,690 cases and 26,120 deaths in 2018) [1]. One in 5 patients develops metastatic (M1) disease. Incidence rates of M1 PCa have increased appreciably although slowly in the past 10 years, with rates among men aged 45–54 years increasing since 2004 [2]. The absolute number of new cases of M1 PCa (annual burden) is projected to increase by 42% over the next decade, with 15,097 new cases expected in 2025. Many questions regarding disease management remain: what is the best way to document and characterize disease burden? Once we have identified and characterized a lesion, what are the best ways to approach these sites of disease? If a primary tumor remains untreated, should it be treated? What should the systemic treatment be in cases of oligometastatic disease? In this review, we will discuss oligometastatic PCa scenarios, and we intend to provide the reader with an evidence-based guide of treatment decisions (Table 1).
Defining Oligometastatic Disease
Clinical Scenario 1
A 55-year-old male with a prostate-specific antigen (PSA) of 33 ng/dL has Gleason 4 + 5 adenocarcinoma of the prostate diagnosed on a biopsy. He is generally well and has no relevant medical history. Computed tomography (CT) and bone scans show evidence of metastasis throughout the pelvis. In your opinion, which terminology best describes M1 PCa in patients who are about to start treatment?
The concept of oligometastatic disease was first proposed by Sam Hellman [3] and Ralph Weichselbaum [4] at the American Society of Clinical Oncology (ASCO) over 20 years ago. It is derived from the Greek word “oligo” meaning “few.” The concept involves controlling a primary tumor completely or controlling a single or limited number of M1 lesions with local therapy, which can result in a cure. Oligometastatic disease is an intermediate biological state with a unique clinical picture within the spectrum of advanced disease, which has a favorable phenotype that is ideal for an intensive approach. This disease state will continue to be redefined as novel imaging tools are adopted as it remains relatively poorly understood. The oligometastatic concept was developed based on data from gastrointestinal-associated liver metastasis: resection of M1 liver lesions in patients with gastrointestinal malignancy is curative. When considering whether a patient has oligometastatic disease, it is important to think about the following: first, if the primary tumor and M1 lesions are controlled and the patient still progresses, the patient did not actually have oligometastatic cancer. Second, oligometastatic cancer treatment should not require any systemic therapy. Lastly, the definition and diagnosis of oligometastatic disease is independent of timing (synchronous vs. metachronous).
The definition of oligometastatic PCa has evolved over time. It was first noted by Soloway et al. [5] that individuals who had a limited number of lesions on bone scans had improved survival outcomes compared to those who presented with bulky disease. This was confirmed in a European study by Ost et al. [6], where individuals who presented with a single M1 site had significantly improved 5-year survival outcomes compared to those who had polymetastatic disease. Gandaglia et al. [7] demonstrated that the survival rates varied depending on the site of disease and the number of metastases in the 3,857 patients presenting with M1 PCa. Visceral metastases alone or with concomitant bone involvement predicted the worst survival outcome compared with bone metastases alone. A retrospective cohort of 436 patients with M1 castration-sensitive PCa (mCSPC) categorized patients into 2 groups at the start of androgen deprivation therapy (ADT): those with de novo disease or prior local therapy and those with high volume or low volume of disease [8]. De novo/high-volume patients showed a robust, >2-fold higher risk of developing castration-resistant PCa (hazard ratio [HR] = 2.09; 95% CI: 1.63–2.66) or death (HR = 2.48; 95% CI: 1.83–3.36). The results from Sridharan et al. [9] indicated that at the time of the first M1 diagnosis, patients with oligometastatic bone disease at either 1 site only or at 2–3 sites experienced longer time intervals to PCa-specific death than patients who were diagnosed with 4 or more bone metastases. Our modern take on oligometastatic PCa came out of an analysis from the SWOG 8494 study, from which researchers began to provide some granularity to this definition [10]. In SWOG 8494, patients with low-volume disease were defined as those with axial metastasis and/or lymph node metastasis only, versus high-volume disease, which included patients with appendicular metastases and/or visceral metastases. Presently, there are a tremendous number of ongoing and retrospective trials that use various definitions for oligometastatic disease. There is no true consensus on the definition, but most ongoing studies define oligometastatic PCa as having a limited number of M1 sites (Fig. 1). Typically, these studies include fewer than 5 lesions; exclude liver, lung, and brain lesions; and include lesions contained within the axial versus the appendicular skeleton [11, 12]. In this case, the patient was diagnosed with de novo M1 PCa and met “low-volume” criteria.
Metastasis-Directed Therapy: Rationale
Clinical Scenario 2
A patient was treated with a radical prostatectomy 6 years ago and underwent salvage radiation 4 years ago. Nine years later, the patient developed a bone metastasis in a rib, which was biopsy-proven. His PSA was 8.74 ng/mL. He was treated with radiation therapy to the left posterior ninth rib plus 1-year of combined ADT. After stopping the hormonal therapy, the patient’s PSA never recurred. He is now 59 years old with hypertension but is otherwise healthy with no other significant comorbidities.
Oligometastatic recurrence following local treatment of PCa is an increasingly frequent disease finding. Oligoprogression is the most common disease state captured by most studies today. If oligoprogression is a pathway to wide-spread M1 disease, then local therapies can alter this path and may slow the disease course. Early ablation of tumors will delay adverse outcomes, including development of symptoms, especially with skeletal metastases for bone-only disease. Early intervention delays the development of castration resistance, as well as the need to start or continue systemic therapies. One example in the radiation realm is stereotactic ablative radiation, also known as stereotactic body radiation therapy (SABR/SBRT). This technique involves highly focused radiation concentrated on limited-volume tumors, with the goal of applying minimal radiation to the surrounding tissue. Four prospective trials have been published in this population. In a multicenter randomized phase II study, patients with oligometastatic PCa were randomly assigned (1:1) to either surveillance (no intervention) or to metastasis-directed surgery or SABR. The median ADT-free survival was 13 months for the surveillance group and 21 months for the metastasis-directed therapy group (MTD) (HR, 0.60, log-rank p = 0.11) [13]. The 5-year ADT-free survival was 8% for the surveillance group and 34% for the MDT group (HR = 0.57, log-rank p = 0.06). The 5-year castration-resistant PCa-free survival was 53% for the surveillance group and 76% for the MDT group (HR 0.62; log-rank p = 0.27). Prostate cancer-related mortality is low within the first 5 years of diagnosis of oligorecurrent PCa. The ORIELE study randomly assigned patients (2:1) to either observation only or to receive SABR to M1 sites outside of the prostate. Progression was observed in 19% of patients treated with SABR versus in 61% of those in the observation arm (p = 0.005) [14]. Treatment with SABR improved the median progression-free survival (PFS) (not reached vs. 5.8 months; HR, 0.30; 95% CI, 0.11–0.81; p = 0.002). In a prospective clinical trial, 33 patients with oligometastatic PCa received SABR to a total of 50 oligometastases [15]. Twenty patients had bone-only disease, 12 had node-only disease, and 1 had mixed disease. Over one-third of patients did not progress and were free from ADT at 2 years. The SABR-COMET study randomly assigned patients (1:2) to receive either palliative standard of care treatments alone (control group) or standard of care plus SABR to all M1 lesions (SABR group) [16]. The majority of these patients had 1–3 sites of disease. In a 2:1 randomization, the median OS was significantly improved for patients who underwent SABR compared to palliative approaches. The median OS was 28 months in the control group versus 41 months in the SABR group (p = 0.090). A nearly 2-fold greater rate of patients were alive in the SABR arm at 5 years: 46% in the SABR arm versus 24% in the control group. Most notably, the SABR patients maintained their quality of life. When imaging identifies exclusive nodal recurrent PCa, salvage lymph node dissection can be a safe MDT option in nodal recurrence after primary treatment. However, the oncological impact of salvage lymph node dissection assessed by strong clinical endpoints remains uncertain [17]. The benefit of metastasis-directed surgery and radiotherapy (RT) to asymptomatic sites of extrapelvic disease is under active investigation and is currently somewhat unclear. In this case, the patient does meet “oligorecurrent disease,” and both systemic and local treatment of all lesions is generally recommended in this scenario.
Treatment of the Primary Tumor in the Setting of Oligometastatic Disease
Clinical Scenario 3
A 55-year-old male has lower back pain. A subsequent PSA is found to be 35 ng/mL. Biopsy revealed Gleason 8 PCa in 6 of 6 cores. A whole-body MRI and bone scan demonstrated a few retroperitoneal lymph nodes and 2 bone metastases in the ribs and pelvis. This patient is healthy with no significant comorbidities. What is the most appropriate next step in the care of this patient?
Cytoreductive treatment in M1 PCa refers to control of the primary tumor by radical prostatectomy, RT, or via metastasectomy at any disease site. There is also discussion surrounding the abscopal effect, a systemic antitumor immune response that reflects the regression of nonirradiated M1 lesions that are at a distance from the primary site of irradiation. It is thought that radiation induces an increased immune response via increased antigen presentation. What is unique about radiation, however, is its synergy with ADT, which increases vascularization. In the tumor microenvironment, where hypoxia is a known mechanism of radiation resistance, increased vascularization can be an incredibly powerful benefit. Androgen signaling can also decrease tumor DNA repair. DNA repair in the tumor is another mechanism of radiation resistance, so ADT helps the effects of radiation. A total of 8,185 M1 PCa patients were identified with the following treatment histories: no surgery or radiation therapy (n = 7,811), radical prostatectomy (n = 245), and brachytherapy (n = 129) [18]. Patients who had surgery and/or radiation had a lower cancer-specific mortality rate than patients who received no local therapy. The 5-year OS and disease-specific survival were each significantly higher in patients undergoing radical prostatectomy (67.4% and 75.8%, respectively) or RT (52.6 and 61.3%, respectively), than those in patients who had no surgery or radiation therapy (22.5% and 48.7%, respectively) (p < 0.001). Rusthoven et al. [19] used the National Cancer Database to evaluate the OS of men with M1 PCa treated with ADT with and without prostate RT. Over 6,382 men with M1 PCa were selected, including 5,844 patients (91.6%) receiving ADT alone and 538 (8.4%) receiving ADT and RT. A total of 69 patients with M1 PCa treated with prostatectomy plus ADT were also identified in this study [19]. In this large contemporary analysis, men with M1 PCa who received prostate RT and ADT lived substantially longer than men treated with ADT alone. Radical prostatectomy was associated with a 52% decrease in the risk of PCa-specific mortality. Intensity-modulated radiation therapy was associated with a 62% decrease in the risk of prostate-specific cancer mortality.
The 2 most prominent studies that were done prospectively to analyze this issue are the HORRAD trial and the STAMPEDE trial. The HORRAD trial is a multicenter, randomized trial that recruited 432 patients with primary bone M1 PCa. Patients were randomized to either ADT with EBRT (RT group) or to ADT alone (control group) [20]. There was no maximum number of metastases as part of the exclusion criteria, and this was not an oligometastatic-only trial. This trial compared ADT to ADT with EBRT to the prostate, in patients with primary bone M1 PCa, and it did not show a significant difference in OS. The median OS was 45 months (95% CI, 40.4–49.6) in the RT group and 43 months (95% CI: 32.6–53.4) in the control group (p = 0.4). Furthermore, there were no subgroups with a statistically significant improvement, whether analyzed by Gleason score stage, age, or number of lesions. The HORRAD trial was negative, but RT was only 70 Gy, which is considered a low dose, and only the prostate was treated not the nodes. Patients had incredibly heavy tumor burdens with PSA >20 ng/mL and high-volume disease. The STAMPEDE RT trial randomized 2,061 men with newly diagnosed M1 PCa. Prostate RT was given in combination with hormonal therapy with or without docetaxel versus the standard of care, ADT alone [21]. Patients were divided into 2 groups. The first group was considered to have a high M1 burden and included patients with at least 1 extra-axial lesion and/or the presence of visceral metastases and at least 4 bone lesions. All other assessable patients were considered to have low M1 burden. RT improved failure-free survival but not OS. RT improved failure-free survival and the 3-year OS in the low-burden group, whereas RT had no benefit in the high-burden group. The low-burden group did have an OS benefit. The meta-analysis aimed to assess the effects of adding prostate RT to ADT in men with mCSPC. There was no evidence that the addition of prostate RT to ADT improved survival (HR = 0.92, p = 0.78). The PFS results based on all men included in the study also did not provide clear evidence that prostate RT extended PFS (HR = 0.94, p = 0.238) [22]. The Advanced Prostate Cancer Consensus Conference (APCCC) guidelines of 2019 showed that the majority of expert panelists do consider radical local therapy to be appropriate for newly diagnosed oligometastatic PCa [23].
A total of 23 patients with M1 PCa (with 3 or fewer bone lesions) undergoing cytoreductive radical prostatectomy (CRP) were compared to 38 men with M1 PCa treated with ADT without local therapy [24]. Clinical PFS and cancer-specific survival were improved with CRP, and CRP effectively prevented complications of the lower and upper urinary tract. In a prospective series, 43 PCa patients with low-volume bone metastases (1–3 lesions) undergoing CRP were compared to 40 patients receiving the best systemic therapy [25]. There was no significant oncological benefit found in the CRP group (overall and castrate resistance-free survival). There was, however, a significant reduction in locoregional complications for patients undergoing CRP (7% vs. 35%). A retrospective case series comprising 106 patients with newly diagnosed M1 PCa examined perioperative outcomes [26]. CRP for men with locally resectable, distant M1 PCa appeared safe and feasible. Complication rates related to CRP were not higher than when radical prostatectomy was performed for standard indications, and CRP avoided complications related to local progression. RT to the primary tumor is emerging as a promising treatment option in low-volume mCSPC. Surgery, however, is not yet a valid option. Continued assessment of the genomic and clinicopathological characteristics to further refine subsets will be important.
Advanced Imaging Will Improve Outcomes in Patients with Low-Volume M1 Disease
Clinical Scenario 4
A 54-year-old male has newly diagnosed high-grade PCa. His screening PSA was 15.6 ng/mL, and his staging scans showed an isolated solitary bone metastasis. We recommended systemic therapy alone, thinking this patient has M1 disease. A prostate-specific membrane antigen (PSMA) positron-emission tomographic (PET) imaging was done, which showed uptake in the actual tumor, but no uptake in the bone island. This suggested that perhaps it was not true bony disease and that this patient was not oligometastatic and can potentially be cured. He underwent a radical prostatectomy and remains with an undetectable PSA.
It is important to remember that conventional imaging is used to define the oligometastatic state in retrospective studies and in most contemporary studies. The National Comprehensive Cancer Network (NCCN) defines staging guidelines for newly diagnosed PCa and suggests that individuals who present with unfavorable intermediate-risk PCa or high- or very-high-risk PCa should be staged with a conventional bone scan, CT, and MRI. More recently, the NCCN has included PET-based imaging for better resolution and more accurate diagnostic staging of the bone. However, the inclusion of PET-based imaging in the NCCN guidelines and in most clinical practice guidelines is limited to imaging for recurrences (Fig. 2) [27-32]. Thus, PET-based imaging is recommended for individuals who have had their primary PCa treated with surgery or radiation and subsequently developed a recurrence. With the increased sensitivity of novel imaging, the rate of diagnosis of oligometastatic disease has increased. A meta-analysis that focused on the impact of gallium-68-PSMA on the management of PCa patients with biochemical recurrence showed that it altered the management of more than half of PCa patients with a biochemical recurrence [33]. It is important to factor in this increased specificity into diagnoses and to remember that some patients who we think have metastases may not. In the clinical scenario above, the patient does meet M1 disease criteria with conventional imaging, but gallium-68-PSMA does not show M1 disease. It is essential to take into consideration the clinical history of the patients to interpret the imaging results. PSMA PET will soon be a tool used in treatment decision-making for most disease stages.
Advances in PET offer greater detection for PSA-recurrent disease. PET, positron-emission tomography; PSA, prostate-specific antigen.
Advances in PET offer greater detection for PSA-recurrent disease. PET, positron-emission tomography; PSA, prostate-specific antigen.
Considerations for Systemic Therapy in Optimal Management of Oligometastatic Disease
Clinical Scenario 5
A 73-year-old male presented with PCa, but there are negative findings on conventional imaging. He has Gleason 4 + 4 PCa diagnosed in 6 of 12 cores on a recent biopsy. His PSA was 13.99 ng/mL prior to the biopsy. He has hypertension and is otherwise healthy and takes no other medications. He is treated with 24 months of combined ADT and 78 Gy intensity-modulated radiation therapy. At 12 months, the PSA is 0.8 ng/mL; at 18 months, 2.6 ng/mL; at 24 months, 3.2 ng/mL; and now at 30 months, following intensity-modulated radiation therapy, the PSA is 5.4 ng/mL. Restaging CT and a bone scan demonstrated M1 disease in 3 ribs, the sternum, and the scapula. The patient remains asymptomatic and otherwise in excellent health. What would the next most appropriate step be in the treatment of this patient?
M1 PCa is a different biological entity than a primary tumor [34]. Primary PCas often harbor multiple morphologically and clonally distinct tumor foci, even though after treatment many subclones are eliminated [35-37]. The lethal clones that were present in the primary tumor will persist in the metastases. MDT may delay ADT, but outcomes are likely better with ADT as ADT in combination with radiation helps to more effectively treat the primary or M1 tumor. ADT is also likely treating the occult M1 disease that we cannot detect with current technologies. The European Organization for Research and Treatment of Cancer (EORTC) did a randomized phase 3 trial assessing the benefit of adding long-term androgen suppression with a luteinizing hormone-releasing hormone agonist to external irradiation in patients with PCa with high M1 risk [38]. The 10-year clinical disease-free survival was 23% in the RT alone group and 48% in the combined treatment group (HR 0.42, p < 0.0001). The ten-year OS was 40% in patients receiving RT alone and 58% in those randomized to the combined treatment (HR 0.60, p = 0.0004). The 10-year prostate cancer mortality was 30% and 10%, respectively (HR 0.38, 95%, p < 0.0001). This is commonly touted as a way to improve radiation therapy, but really, it is a way to attain systemic control of the disease. When ADT plus radiation is given for localized disease in the high-risk setting, the combination could help reduce the primary tumor volume and help achieve systemic control of the disease. Thus, ADT is the standard of care for men with locally advanced PCa. ADT is the standard of care in M1 PCa, whether given as ADT alone or as ADT with abiraterone or chemotherapy. Some patients with oligometastatic PCa show rapid progression at multiple sites, some progress slowly, and others never progress. A surveillance strategy is the therapeutic recommendation in the absence of symptoms and an observed slow rise in PSA levels, whereas androgen suppression is the recommendation in the case of rapidly progressive and symptomatic disease. Furthermore, docetaxel-based chemotherapy combined with ADT has demonstrated superiority in randomized trials. However, treatment toxicity and reduction in quality of life are main issues when treating patients with oligometastatic disease. Many questions remain regarding the management of patients that present with nonsymptomatic oligometastatic PCa. Treatment for these patients would follow the paradigm that is currently most prominent in PCa oncology: integrate new therapies in earlier stages of disease rather than reserving them for the later stages [39]. With docetaxel, there was no OS benefit in low-volume patients in the CHAARTED study. In fact, in the APCCC guidelines of 2019, a consensus panel of experts suggested that docetaxel for oligometastatic disease was not essential and should not be considered a vital component of the treatment [40]. In the LATITUDE trial involving men with high-risk, newly diagnosed mCSPC, the rate of OS was significantly higher among those who received ADT plus abiraterone and prednisone than among those who received ADT plus placebo (median survival 53.3 vs. 36.5 months, HR 0.66, 95% CI 0.56–0.78) [41]. In the STAMPEDE study, the addition of abiraterone to ADT did confer a significant advantage in terms of PFS and OS, even in patients with lower volume disease [42]. The STAMPEDE study demonstrated that the M1 status did confer some degree of importance in terms of the outcome in patients receiving ADT plus abiraterone versus ADT alone. This suggests that in many patients with lower volume disease, the addition of abiraterone may be beneficial. In later analyses of men with mCSPC, coadministration of abiraterone with ADT was beneficial irrespective of “risk” or “volume” [43-45]. Regulators and purchasers could consider extending the approval of abiraterone in M1 PCa to all patients. ARCHES is a multinational, double-blind phase III trial in which 1,150 patients with mCSPC were randomized (1:1) to enzalutamide plus ADT or placebo plus ADT, stratified by disease volume [46]. Enzalutamide plus ADT significantly reduced the risk of radiographic disease progression or death by 61% compared with placebo plus ADT (HR, 0.39; 95% CI, 0.30–0.50; p < 0.001). The treatment effect of enzalutamide plus ADT was independent of low-volume disease and prior docetaxel chemotherapy. The double-blind phase III TITAN clinical trial in mCSPC randomly assigned patients to ADT plus either apalutamide (240 mg daily) or placebo [47]. The benefit was seen both in men with high-volume and low-volume M1 disease. An ongoing study, the Alliance Foundation trial (AFT-19), is enrolling patients with rising PSA after radical prostatectomy with no metastases on conventional imaging [48], as well as patients who have low-volume pelvic lymph node disease and patients who have PSMA PET evidence of M1 disease. In this study, a small number of patients will be treated with systemic therapy alone as there are 3 arms: 1 is ADT alone, 1 is ADT plus apalutamide, and the third is ADT plus apalutamide plus abiraterone. The addition of apalutamide with or without abiraterone acetate/prednisone, compared with ADT alone, will prolong disease suppression and potentially eradicate micrometastatic disease with a finite duration of treatment in patients with biochemically relapsed PCa. When considering systemic therapies, we should consider oligometastatic disease as having a different biology than the more advanced M1 cases. We do not advocate for docetaxel in this setting as it has not been proven to be beneficial. However, as emerging studies are showing, abiraterone, enzalutamide, apalutamide, and perhaps darolutamide, as well as other drugs, may be applicable in the oligometastatic disease setting (PFS, OS, and QOL) (Fig. 3). Treatment decision-making for PCa is complex for both patients and physicians, so it is essential to consider the individual patient and the different disease characteristics.
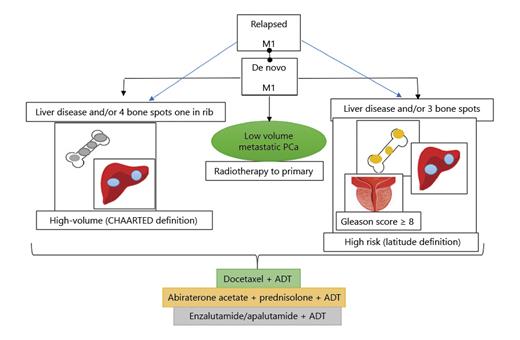
Clinical drivers of decision-making. PCa, prostate cancer; M1, metastatic; ADT, androgen deprivation therapy.
Clinical drivers of decision-making. PCa, prostate cancer; M1, metastatic; ADT, androgen deprivation therapy.
Conclusion
Oligometastatic disease is not a new concept, but it is a “new” clinical state and remains relatively poorly understood. This idea elicits great interest and hope among clinicians, researchers, and patients. Finally, there is very little data to guide treatment decisions when considering oligometastatic PCa. Oligometastatic disease will be redefined as novel imaging tools continue to be adopted as it is an individual, heterogeneous entity with distinct M1 phenotypes and wide prognostic variability. Future studies should aim to provide clinicians with guidance on how to better tailor personalized treatment regimens.
Statement of Ethics
This study does not involve ethical issues.
Conflict of Interest Statement
All authors declare no conflict of interest.
Funding Sources
No funding received for this work.
Author Contributions
B.C. contributed to the research conception and design. B.C. and C.W. contributed to the data analysis and interpretation. C.W and O.G. drafted this paper. O.G. supervised the study. All authors approved the final version of this work.