Abstract
Purpose: Bacille Calmette-Guerin (BCG) is considered the most effective agent for non-muscle invasive bladder cancer (NMIBC). However, due to BCG-related toxicity, multiple studies have suggested the role of newer chemotherapeutic drugs. The aim of our study was to evaluate intravesical gemcitabine + docetaxel (Gem/Doce) versus BCG with respect to quality of life (QOL), safety, and efficacy in NMIBC. Methods: A total of 60 patients with NMIBC were evaluated between July 2019 and December 2020 in a prospective manner. The sample size calculation was done, keeping in mind the incidence of intravesical BCG-related complications of up to 50–60% and 20–30% for Gem/Doce combination. The p value of 0.05 was kept as statistically significant. The enrollment ratio was kept at 1, and power of study was aimed at 80%. The study population was alternatively assigned to two groups (BCG vs. Gem/Doce) of 30 patients each. Both the groups received 6 weekly doses of induction therapy followed by 6 monthly doses of maintenance therapy if no recurrence was noted at interim follow-up. QOL scores, safety, and efficacy were assessed at beginning of intravesical therapy, end of induction, and 6 months of maintenance therapy. Cystoscopy examination and cytology were performed at the end of induction therapy and 3-monthly thereafter. Result: The preliminary results at the end of 6 months following maintenance therapy showed that the demographic profile, histological stage, and grade were comparable between two groups. The QOL scores using QLQ-30 and QLQ-BLS-24 showed statistically significant differences with the Gem/Doce arm showing better outcomes. There were no progressions to higher stage, while one recurrence each was seen in both groups. Patient-related side effects measured by CTCAE (Common Terminology Criteria for Adverse Events)version 5 showed that the BCG group had higher toxicity profile as compared to Gem/Doce group. Conclusion: Gem/Doce combination intravesical therapy is a promising alternative to BCG for treatment of NMIBC, showing better QOL measures and lesser side effects.
Introduction
Intravesical Bacillus Calmette-Guerin (BCG) is the first-line option in the management of carcinoma in situ and the high-risk disease non-muscle invasive bladder cancer (NMIBC) [1], while in intermediate-risk NMIBC, both BCG and intravesical chemotherapy are accepted alternatives. Among the adjuvant options, superiority of BCG has been only established for disease recurrence but not progression and it needs to be balanced against higher toxicity [2]. Gemcitabine (Gem) has an excellent toxicity profile and promising efficacy in NMIBC, including those at high risk of disease recurrence [3‒5]. Gem has shown its efficacy even in BCG refractory disease with a significant decrease in recurrence [6]. Furthermore, addition of docetaxel with Gem has shown complete response in various types of BCG failure [7]. We prospectively evaluated intravesical BCG versus sequential intravesical Gem + Doce in terms of quality of life (QOL), safety, and efficacy in NMIBC.
Material and Methods
This prospective pilot study was conducted at the Post Graduate Institute of Medical Education and Research, Chandigarh, India, between July 2019 to December 2020. After approval from the Ethical Committee (NK/5750/M. Ch/538), a total of 60 patients were enrolled in the study. The sample size was calculated keeping in mind the incidence of intravesical BCG-related complications up to 50–60% and 20–30% for Gem + docetaxel (Gem/Doce) combination. The p value of 0.05 and less was kept as statistically significant. The enrollment ratio was kept at 1, and power of study was aimed at 80%. All patients of intermediate- and high-risk NMIBC were enrolled in the study protocol. The exclusion criteria were as follows: concomitant upper tract urothelial cancer, history of prior hypersensitivity to docetaxel/Gem or BCG, neurogenic bladder dysfunction, patients who have received any intravesical therapy for NMIBC within the last 6 months, pregnant and lactating women, patients with severe hepatic dysfunction or renal impairment, patient opting to undergo early cystectomy, active urinary tract infection, and genitourinary tuberculosis.
All the patients with urinary bladder mass underwent transurethral resection of bladder tumor (TURBT) following standard surgical technique. After histopathological confirmation of NMIBC, patients were alternatively assigned to receive 6 weekly cycles followed by 06 monthly cycles of intravesical BCG or Gem/Doce. Written informed consent was taken from all the patients and full information was explained regarding efficacy and side effects of both BCG as well as Gem + Doce therapy.
The induction phase of weekly 6 intravesical instillations was started 2 weeks after TURBT. After emptying the urinary bladder, in group one, 120 mg BCG (Danish 1331) diluted in 50 mL of saline was instilled in the urinary bladder using 10 French infant feeding tube under gravity and the patients were advised to hold urine for 90 min after instillation. Similarly, in group two, 2 g Gem diluted in 50 mL of saline was instilled in the urinary bladder, and patients were asked to hold urine for 90 min. Patients were then asked to empty the bladder and 40 mg docetaxel diluted in 50 mL of saline was instilled. The patients were advised to hold urine for the next 60 min. All intravesical instillations were carried out under strict aseptic precautions. Maintenance schedule in both groups consisted of 06 monthly instillations of BCG or Gem + Doce combination in groups 1 and 2, respectively. The maintenance phase in both the groups started 2–4 weeks post induction therapy and followed same dosage and methodology.
Patients were assessed at start (D0 – before instillation for assessment of baseline data), end of induction therapy (D1), and at end point of this study, i.e., 6 months following start of maintenance intravesical therapy (D2). The QOL assessment was made using two questionnaires: the European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30 version 3.0 (EORTC QLQ-C30) [8] and the EORTC Quality of Life Superficial Bladder Cancer-Specific (EORTC QLQ-BLS-24) [9]. The QLQ-C30 is a validated and integrated tool for measurement of cancer-related QOL Overall. It consists of 15 items that are scored on 4-point scales. The higher the score more is the QOL disruption for the patient. Bladder cancer-specific scale (QLQ-BLS-24) is used to evaluate symptoms specific to urological practice. The scale includes urinary tract symptoms, intravesical treatment problems, future perspective, abdominal bloating and flatulence, and a score for sexual functioning. Cystoscopy and cytology were performed at end of induction phase and every 3 months thereafter till the end of the study. In case of a recurrence, TURBT was performed, and based on histopathology, decision to continue on maintenance therapy or start repeat induction versus early cystectomy was taken. Upstaging from Ta to T1, low grade to high grade, or any occurrence of carcinoma in situ or T2 at bladder biopsy or TURBT was defined as progression. Complications were graded according to CTCAE (Common Terminology Criteria for Adverse Events) version 5.0 [10] (online suppl. File; for all online suppl. material, see www.karger.com/doi/10.1159/000524098). All the patients completed the study trial and there was no dropout.
The data were analyzed using descriptive and inferential statistics. The mean and standard deviation were computed using conventional statistical formulas. The χ2 test was used to assess for statistical significance in the two groups for demographic and QOL parameters. Student’s t test was used for analysis of complication at all assessment points (D0, D1, and D2). The χ2 test was used to assess statistical significance of individual parameters in the two QOL scales. Statistical analysis was conducted using IBM R SPSS-software 20.0. Statistical significance was defined as p value <0.05.
Result
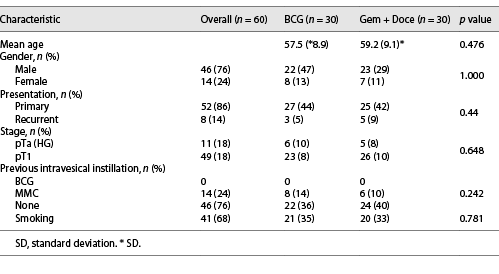
The patient characteristics are listed in Table 1. The mean age of patients in group 1 (BCG) was 57.5 (8.89) years and group 2 (Gem + Doce) was 59.17 (9.1) years. There was a male predominance overall with 46 males and 14 females. Smoking was seen in about 70% of the study population. Based on primary or recurrent presentation, both the groups were comparable with majority diagnosed with primary NMIBC. Forty-nine out of 60 patients had T1 disease on histopathology. The rest 11 patients had Ta high-grade disease. The two groups were comparable based on grade and stage of the disease. With respect to previous intravesical instillation, none of the patients received BCG in the past. Eight patients in group 1 and six in group 2 received a single dose 40 mg intravesical mitomycin immediately after TURBT which was comparable between two arms.
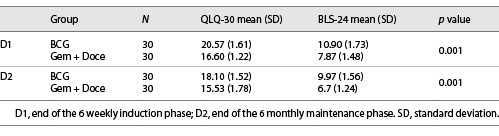
The QOL scores and safety profiles for both the groups were equally matched at baseline (D0). The QOL scores in group 2 (Gem + Doce) for both questionnaires were better at assessment points (D1 and D2) than group 1 (BCG) and the difference in scores was statistically significant (p value 0.00) (Table 2). In subset analysis, functional scale, global health scale, and pain score in QLQ-30 showed statistically significant difference in favor of patients receiving Gem/Doce. In cumulative analysis of BLS-24 scale, urinary symptoms were significantly less in group 2 as compared to group 1 (p value 0.00). Other individual parameters like intravesical treatment-related problems, flatulence and bloating, future prospective and sexual well-being were reported to be better in group 2; however, statistical significance could not be reached.
There were no grade 3 and above complications noted in either groups receiving intravesical therapy. Dysuria and bladder spasm were the most commonly recorded complications. Patients receiving BCG (group 1) had significantly higher side effects as compared to patients receiving Gem/Doc each at end of induction and maintenance therapy. Mild hematuria was reported in 1 patient in group 2 and in 8 patients in group 1 at D1. Even grade 2 CTCAE of complications were higher in the BCG group as compared to the Gem/Doc group at assessment points (D1 and D2), and the difference was statistically significant (p value 0.000) (Table 3).
Any evidence of recurrence or progression in NMIBC patients was noted on follow-up cystoscopy examination. There were two recurrences at the end of induction therapy, one each in each group. In both the cases, patients underwent TURBT, and the histopathology of recurrent lesions revealed Ta low-grade lesions. Patient continued to receive maintenance therapy on follow-up. There was no progression of the disease in either group.
Discussion
Since its description by Morales et al. [11], BCG has been the most accepted form of intravesical therapy used in NMIBCs around the world, especially in intermediate- and high-risk disease. Although BCG is superior to chemotherapeutic agents, it is least tolerable among all intravesical drugs [12]. According to an EORTC study, around 20% of patients had to stop the BCG therapy midway due to side effects produced by intravesical instillations [13]. A systematic review of intravesical BCG in NMIBC by Shelly et al. [14] reported cancer recurrence as the most common reason (28.7% of patients) for discontinuing the treatment and another 10% of patients discontinued treatment owing to its toxicity such as dysuria, urinary frequency, and fever. To compound the problem further, the optimal dose and frequency for BCG administration as well as the duration of maintenance instillations are still a matter of debate [15]. Multiple studies have proven that monthly intravesical instillation of BCG for 12 months is equally effective to SWOG regimen of 3-weekly instillations at 6 month intervals for 36 months [16, 17]. This maintenance schedule leads to better balance between efficacy and safety of intravesical BCG instillation and therefore increases patient compliance. Our institutional protocol is at a maintenance phase for 01 year with 12 monthly BCG instillations.
The failure rate for BCG therapy is approximately 40% for 2 years follow-up [18]. The EAU recommendations for BCG-failure patients remain radical cystectomy with urinary diversion. However, it is associated with significant morbidity and mortality as well as dramatic lifestyle changes [19]. For patients keen on bladder preservation or who are poor surgical candidates, alternative intravesical therapy should be offered. Repeat BCG therapy is effective in 1/3rd of patients only [20]. Multiple studies have suggested role of newer drugs for intravesical instillation, but its efficacy remains subject to further research and validation [7].
Gem, a broad-spectrum antitumor drug, incorporates into cellular DNA and inhibits cell growth and increases apoptosis. Intravesical Gem instillation has shown better toxicity profile and promising efficacy in NMIBC [3]. A SWOG study evaluating single-agent intravesical Gem in NMIBC with BCG failure depicted recurrence-free survival rates of 28% at 1 year and 21% at 2 years post-therapy. The therapy was well tolerated with no major side effects reported [21]. A combination of Gem plus mitomycin in BCG refractory NMIBC demonstrated a 50% disease-free rate at 18 months follow-up [22]. Lightfoot et al. [23] demonstrated an initial complete response rate of 68% with recurrence-free survival of 48% at 1 year and 38% at 2 years post-treatment using Gem plus mitomycin in BCG refractory NMIBC.
Docetaxel, a taxane derivative, binds to tubulin component of microtubules and prevents its disassembly resulting in decreased tumor proliferation and cell death. It has higher tissue concentration ability when given intravesically. McKiernan et al. [24] reported first-ever human trial using intravesical docetaxel and concluded 56% efficacy with minimal toxicity in patients with BCG refractory NMIBC. Barlow et al. [25] studied long-term survival outcomes with intravesical docetaxel for recurrent NMIBC after failed BCG therapy and concluded significant efficacy and durability.
The current study shows the preliminary results at the end of 6 months of maintenance phase of intravesical instillations of either BCG or Gem + Doce combination therapy. Gem + Doce intravesical therapy is equally efficacious as BCG for treatment of BCG naive NMIBC, while the long-term outcomes are awaited. A similar study by Steinberg. et al. [20] showed Gem + Doce combination intravesical therapy to be efficacious in 66% of patients when used as salvage therapy for BCG failure cohort. Thomas et al. [26] used intravesical Gem + Doce instillation in BCG naive patients and compared its efficacy and toxicity with BCG retrospectively. They reported good efficacy rates with minimal toxicity in combination chemotherapy group. Our study is unique in this sense that we used Gem + Doce sequential therapy in BCG naive NMIBC patients and achieved better QOL scores, better toxicity profile with equal efficacy rates.
Around 40% of the Indian population is infected with tuberculosis and almost whole of the population is immunized with BCG [27]. This preexisting exposure to BCG may cause accelerated response against tumor cells and increased local inflammation. Niwa et al. [28] reported stronger therapeutic effects of intravesical BCG therapy and potentially major BCG-related side effects in patients with prior BCG exposure. This may be one of the hypotheses explaining increased side effects and poor QOL scores in patients receiving intravesical BCG instillation in our study.
The role of inflammation on therapeutic response in BCG effectiveness is still under research. The study published by Ferro et al. [29] gives us a direction for further research for utilizing inflammatory markers such as Th1 and Th2 along with basophils as immune modulators to assess the response to intravesical BCG. The same author also suggested that type 2 diabetes mellitus is predictive of an increased risk of recurrence and progression in patients with high-grade urothelial cancer. The hypothesis suggests that chronic inflammation in diabetes is a hallmark of carcinogenesis, as a result of insulin, IGF-1, proinflammatory cytokines, oxidative stress, and growth factors effects [30]. If validated, this can be an important tool in management of patients with bladder cancer in near future.
QOL parameters form an important aspect for acceptance of any treatment protocols by the patient. Better QOL scores with a low number of patient-reported side effects in the Gem + Doce group make it a safe and effective option for NMIBC patients, whether BCG naive or with BCG failure. To our knowledge, this is the first study reporting QOL comparison of sequential intravesical Gem + Doce with BCG in NMIBC in the Indian population. Our study being a pilot study lacks randomization and longer follow-up schedule. However, our results for QOL are in accordance with existing literature. Better QOL with safety profile in our study will form the basis for future randomized studies with larger patient numbers to further define this promising option for treatment of NMIBC. The long-term outcomes of this study are awaited. Finally, no study in these times can be exempt from the effect of pandemic. Our study like multiple other studies in world literature [31] does point out towards this effect, which may range from recruitment of patients to providing timely care and follow-up. We strongly recommend that a large-scale multi-institutional study is need of the hour to study this effect on treatment and oncological outcomes of NMIBC.
Conclusion
Sequential Gem/docetaxel is a safe, equally efficacious alternative therapy to intravesical BCG with improved QOL scores in treatment of NMIBC.
Acknowledgment
We sincerely thank Mrs. Kusum for doing statistical analysis during preparation of manuscript.
Statement of Ethics
This study protocol was reviewed and approved by the Post Graduate Institute of Medical Education and Research Ethics Committee. Ref. no NK/5750/M. Ch/538. For the study, written informed consent was taken from all the patients.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
No funding was received during manuscript preparation.
Author Contributions
Tarun Pareek and Santosh Kumar – initial concept, design, and draft. Tarun Pareek and Kalpesh Parmar – collection of data. Aditya Prakash Sharma – revision of manuscript. Kalpesh Parmar and Santosh Kumar – critical comments. All the authors read the manuscript and contributed equally.
Data Availability Statement
All data generated or analyzed during this study are included in this manuscript and its online supplementary material. Further inquiries can be directed to the corresponding author, if needed.