Abstract
Purpose: The purpose of the study was to evaluate the association between alcohol consumption and risk of erectile dysfunction (ED). Methods: PubMed was searched for reports published before June 2019. Data were extracted and combined odds ratios (ORs) calculated with random-effects models. Results: Finally, 46 studies were included (216,461 participants). The results of our meta-analysis indicated that there was a significant association between regular alcohol consumption and ED (OR 0.89, 95% confidence interval [CI]: 0.81–0.97). There was no indication of publication bias (Egger’s test, p = 0.37). In the stratified analysis, the pooled OR of ED for light to moderate and high alcohol consumption was 0.82 (95% CI: 0.72–0.94) and 0.82 (95% CI: 0.67–1.00), respectively. No variable related to the source of heterogeneity was found in univariate and multivariate meta-regression analyses. A dose-response meta-analysis suggested that a nonlinear relationship between alcohol consumption and risk of ED was observed (p for nonlinearity <0.001). Conclusion: A J-shaped relationship between alcohol consumption and risk of ED was observed. Alcohol should be taken in moderate quantities in order to obtain the dual effect of disinhibition and relaxation. If taken chronically, it could provoke vascular damages.
Introduction
Erectile dysfunction (ED) has been one of the most common complaints among men with sexual health issues [1], accurately defined as the persistent inability to attain a satisfactory erection of the penis to permit satisfactory sexual intercourse [2]. It has been estimated that worldwide the prevalence of ED will be 322 million cases by the year 2025 [3]. It is evident that ED has become a measurable health disorder for men globally that requires medical and public health attention. Risk factors, including hypertension, diabetes mellitus, coronary artery diseases, and sociodemographic conditions, has been associated with ED [4]. It is of interest to look at the significant effect of alcohol consumption on improvement of erectile function.
Although drinking is often associated with men’s sexual activity, the findings of studies examining alcohol’s effects on men’s ED remained inconsistent. Animal experiments show that chronic consumption of high doses of alcohol (Male Wistar rats, ethanol: 20% v/v, for 6 weeks) increases the expression of the catalytic subunits of the enzyme nicotinamide adenine dinucleotide phosphate oxidase, which is the main source of reactive oxygen species in the endothelium and vascular smooth muscle cells [5, 6]. Reactive oxygen species induce activation of mitogen-activated protein kinases and production of inflammatory cytokines [7]. Moreover, chronic alcohol consumption (Male Wistar rats, ethanol: 20% v/v, for 6 weeks) increases reactive oxygen species production in the corpus cavernosum, which may contribute to ED [7]. Some epidemiological studies have shown that chronic consumption of high doses of alcohol might exert a damaging effect on ED in general population [8, 9]. However, a few of articles were different from the results of above ones [10, 11], those shown that a high dose of alcohol was not associated with ED.
The light and moderate consumption of alcohol might produce a protective effect on ED in both diabetic men and in general population [12, 13], and partly, the beneficial effects of alcohol on erectile function might be due to the long-term benefits of alcohol on high-density lipoprotein cholesterol and other variables that increased the availability and activity of nitric oxide. Moreover, light to moderate alcohol consumption upregulates paraoxonase 1 expression and serum activity, whereas heavy alcohol consumption had the opposite effects in both animals and humans. Paraoxonase 1 can detoxify the homocysteine metabolism, which can pathologically cause toxic effects on the endothelium [14]. So it was fair to hypothesize that alcohol may demonstrate a J-shaped relationship with ED. Although there were 2 meta-analysis studies and they had summarized the relationship between alcohol consumption and ED, they included a limited number of references and did not demonstrate the threshold level of alcohol consumption exerting a damaging effect on ED. We therefore performed this meta-analysis of the available epidemiological evidence assessing the relationship between alcohol consumption and ED risk.
Materials and Methods
Search Strategy
Based on the hospital’s limited biomedical database, PubMed was searched for reports published from inception to May 2019, with the keywords “alcohol,” “ethanol,” and “Erectile.” The reference lists of identified publications (including reviews) were also searched to identify further pertinent studies.
Selection Criteria
All identified studies were independently reviewed by 2 investigators (S.L. and J.M.S.). Studies, if they had been reported in English, the exposure of interest was alcohol or ethanol, and the outcome of interest was ED, odds ratio (OR) or RR with 95% confidence interval (CI) were provided or data provided allowed their calculation, were included in the present meta-analysis. The largest study was preferred when data were duplicated. Animal studies, reviews, systematic reviews, meta-analyses, or studies without sufficient data were excluded before full-text assessment.
Data Extraction
Data were extracted independently from each study by 2 investigators (S.L. and J.M.S.). The first author’s last name, year of publication, country/region, population sources, design type, definition, age, number of cases and sample size, level of alcohol consumption, OR or RR with 95% CI for each category of alcohol consumption, and details of adjustment for potential confounding factors were recorded.
Statistical Analysis
The Mantel-Haenszel method was used to calculate OR or RR and 95% CI, when studies were not provided. If the incidence of disease is low, RR is approximately equal to OR [15]. The combined effects were estimated by combined OR, which was calculated by combining logarithmic risk estimates, for random-effects models, weighted by the inverse variance method to evaluate the association between alcohol consumption and ED risk. The I2 statistic was used to assess heterogeneity between eligible studies. If I2 > 50%, the heterogeneity was considered to be statistically significant. To explore the source of heterogeneity, we operated meta-regression with covariables, such as region, population sources, definition of ED, adjusted and grade of ED. Subgroup analysis was further operated to assess the effects of the factors which had been identified by meta-regression. The potential publication bias was estimated by Egger’s quantitative test.
The pooled dose-response relationship between alcohol consumption and ED risk was explored by a dose-response meta-analysis. Exposure data were converted into a uniform measurement (drinks/week). If alcohol consumption was reported in drinks/day, we multiplied the consumption by 7. When alcohol consumption was reported in g/day, the alcohol intake was converted into drinks/week assuming that 1 drink contains 12 g of alcohol [16]. The median or mean alcohol consumption was considered as the corresponding exposure dose. If the median or mean consumption was not reported, the midpoint between the upper and lower range was assigned in each category as the median consumption. When the lowest category was open-ended, its lower boundary was set to zero. When the highest category was unrestricted for alcohol consumption, the exposure dose was defined by the lower end value of the category multiplied by 1.2. A median intake of <14 drinks/week was defined as light to moderate alcohol consumption; a median intake of ≥14 drinks/week was defined as high consumption. Nondrinkers and <1 drink/week (concluding: socially, monthly, noncurrent, and abstains) were regarded as the reference group.
A restricted cubic spline model was estimated using generalized least square regression to assess the pooled dose-response relationship between alcohol consumption and ED risk. All statistical analyses were performed using Stata (version 12.0, StataCorp, College Station, TX, USA), statistical tests were 2-sided and used a significance level of p < 0.05.
Results
Search Results and Characteristics of Included Studies
A total of 46 studies including 216,461 participants were included (Table 1; Fig. 1, flowchart of study selection). 41 were cross-sectional studies [9‒11, 13, 17‒53], 3 were prospective studies [8, 54, 55], 1 was case-control study [56], and 1 was longitudinal study [57]. Thirty eight studies used “none”/“never”/“nondrinker”/“0 drinks/week” as the control group, 8 studies used “noncurrent”/“abstains”/“socially”/“monthly”/“≤1 drink/month”/“<1 drink/week”/“<1 drink/day”/“<2 drink/day” as the control group. Among the studies, 12 were conducted in Europe, 22 in Asia, 6 in South America, 5 in North America, and 1 was multinational. ED was assessed with the International Index of Erectile Function-5 (IIEF)-5 in 15 studies, with IIEF-15 in 8 studies, and through other methods in 23 studies. All of original studies included in this meta-analysis evaluating ED were blinded to consumption of alcohol.
Alcohol Consumption and Risk of ED
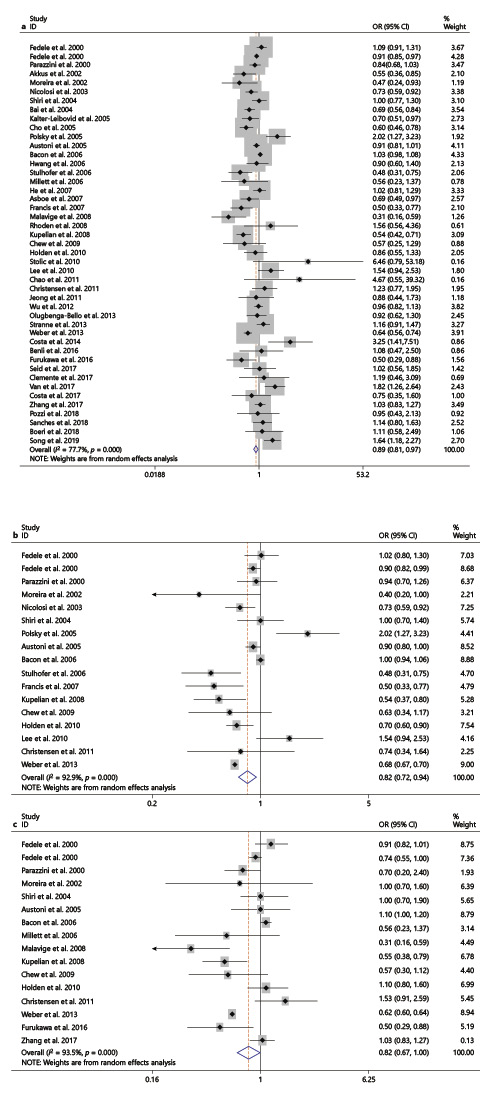
Based on the criteria of reference group, 44 studies about regular alcohol consumption versus reference group were included [8‒11, 13, 17‒19, 21‒54, 56, 57]. Overall, there was significant association between regular alcohol consumption and ED (OR 0.89, 95% CI: 0.81–0.97; I2 = 77.7%, Fig. 2a). There was no indication of publication bias (Egger’s test, p = 0.37). In the stratified analysis, the pooled OR of ED for light to moderate and high alcohol consumption were 0.82 (95% CI: 0.72–0.94; I2 = 92.9%) and 0.82 (95% CI: 0.67–1.00; I2 = 93.5%), respectively (Fig. 2b, c).
Forest plots of the association between ED and regular alcohol consumption (a) and light to moderate alcohol consumption (b) and high alcohol consumption (c). OR, odds ratio; CI, confidence interval; ED, erectile dysfunction.
Forest plots of the association between ED and regular alcohol consumption (a) and light to moderate alcohol consumption (b) and high alcohol consumption (c). OR, odds ratio; CI, confidence interval; ED, erectile dysfunction.
In the subgroup analysis, the pooled OR of ED for regular alcohol consumption versus reference group was associated with a decreased risk of ED in population-based group (OR 0.87, 95% CI: 0.78–0.98), in definition of other methods about ED group (OR 0.86, 95% CI: 0.77–0.98), in the “yes” of adjusted group (OR 0.88, 95% CI: 0.81–0.97), in moderate/complete ED group (OR 0.77, 95% CI: 0.63–0.95), and in complete ED group (OR 0.60, 95% CI: 0.43–0.84). However, the similar relation could not be found in other groups (Table 2).
The pooled OR of ED for light to moderate alcohol consumption versus reference group [8, 9, 17‒19, 22, 24, 27, 29, 32, 35, 36, 38, 40, 54, 56] was associated with a decreased risk of ED in Europe group, in Asia group, in South America group, in population-based group, in definition of IIEF-5 and other methods about ED groups, in the “Yes” of adjusted and “no” of adjusted groups, in moderate/complete and complete ED groups. However, the similar relation could be found only in Asia group for high alcohol consumption versus reference group (Table 2) [8‒11, 17‒19, 24, 26, 32, 34, 35, 38, 40, 54]. No variable related to the source of heterogeneity was found in univariate and multivariate meta-regression analyses (data not shown).
Dose-Response Meta-Analysis
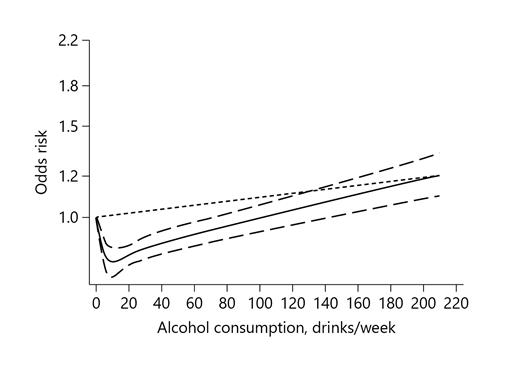
Overall, 17 studies were included in final dose-response meta-analysis [10, 11, 17, 18, 20, 22, 24, 26, 27, 29, 34‒36, 40, 54‒56]. The nonlinear relationship of alcohol consumption with ED was observed (p for nonlinearity <0.001; Fig. 3). Compared with never/lowest drinkers, the pooled ORs were 0.87 (95% CI: 0.83–0.91), 0.81 (95% CI: 0.76–0.86), 0.82 (95% CI: 0.78–0.87), 0.95 (95% CI: 0.90–1.00), and 1.08 (95% CI: 1.01–1.16) for coffee consumptions of 4, 11, 14.5, 73, and 145.5 drinks/week, respectively.
Dose-response analysis between risk of ED and alcohol consumption with restricted cubic splines in a random-effects dose-response model. The solid line and the dashed long line represent the estimated relative risk and its 95% CI. The dashed short line represents the linear relationship. ED, erectile dysfunction; CI, confidence interval.
Dose-response analysis between risk of ED and alcohol consumption with restricted cubic splines in a random-effects dose-response model. The solid line and the dashed long line represent the estimated relative risk and its 95% CI. The dashed short line represents the linear relationship. ED, erectile dysfunction; CI, confidence interval.
Discussion
People may use alcohol and other substances to tackle sexual performance anxiety, enhance sexual performance, or overcome sexual dysfunction. A World Health Organization study for alcohol and high-risk sexual behavior reported that 12% males in the general population consumed alcohol prior to first sexual intercourse due to perceived positive effect of alcohol to improve sexual pleasure. Furthermore, alcohol was commonly used prior to intercourse with commercial sex worker. However, in the long run, substance abuse could impact on sexual functioning negatively and may lead to the onset of sexual disorders [58].
The present meta-analysis, involving 216,461 participants from 46 studies, has evaluated the association between ED and alcohol consumption. Overall, it has shown that regular alcohol consumption was significantly associated with a reduced risk of ED. The results of this study are also consistent with a previous meta-analysis [59]. However, it was different from the other one [60]. In the stratified analysis, the present meta-analysis showed that light to moderate alcohol consumption was correlated with a decreased risk of ED, instead of high alcohol consumption. Furthermore, a nonlinear association of dose-response analysis was further found between alcohol consumption and ED risk, and the risk increased quickly in very heavy alcohol consumption level. A J-shaped relationship between alcohol consumption and risk of ED was observed. Based on the previous studies, we inferred that alcohol may be a 2-side sword. On the one hand, alcohol should be taken in moderate quantities in order to obtain the dual effect of disinhibition and relaxation. On the other hand, alcohol abuse can have lasting effects on the liver, leading to increased levels of estrogen and low levels of testosterone, both of which can contribute to ED [35, 61, 62]. It should be noted that nondrinkers were not the group with the lowest risk of ED, which might contribute to that nondrinkers might be formerly heavy drinkers and had quit due to some disease [63].
Significant heterogeneity was observed in this study, to explore the source of heterogeneity, meta-regression analysis and subgroup analysis were performed. However, the heterogeneity in results of subgroup analysis was still high, and no variable related to the source of heterogeneity was found in univariate and multivariate meta-regression analyses. One possible explanation may be the fact that as the residual confounders for meta-analysis of epidemiological studies are inevitable, although the adjusted measurement whenever available was applied, the effects of confounding could not be excluded completely. For example, the majority of cross-section studies might induce more recall and selection biases. Besides, wide range of age in included studies could also impact the heterogeneity. It is noted that the complete ED groups were significant in all of comparative groups, the studies included in the analysis were adjusted by confounding, on some level, the protective association between alcohol consumption and ED might be possible.
Several strengths could be highlighted in this study. First, the present meta-analysis provides the recent and most complete evidences on the association between ED risk and alcohol consumption. Second, the combined use of categorical meta-analysis and dose-response analysis can provide more information [64]. Moreover, the result of Egger’s test did not support the presence of major publication bias.
Except the high heterogeneity, several potential limitations should also be considered. First, confounding was inherent in all observational studies, and some bias in included studies could not be avoided, such as recall and selection biases, which cannot be solved at a meta-analysis level. Because of adjusting for different number of factors from different studies, each study had a different level of confounding. In addition, different categories of alcohol drinking, such as beer, wine, and liquor, had different affections [63]. The uniform measurement of alcohol drinking might cause measurement errors to some extent. Moreover, definitions of ED were skimble-scamble, which load to the different summary estimate for each definition.
Conclusions
The present meta-analysis has indicated that regular alcohol consumption was significantly associated with a reduced risk of ED. Heavy alcohol consumers were not associated with ED risk. A J-shaped relationship between alcohol consumption and risk of ED was observed. But for all those, alcohol should be taken in moderate quantities in order to obtain the dual effect of disinhibition and relaxation. If taken chronically, it could provoke vascular damages. We should explain the results with cautions for all the limitations and further studies with larger sample sizes, as well as greater statistical power would be needed to confirm the conclusions.
Statement of Ethics
As our research focused on summarizing relationships and did not include animal experimental research or human body research, we considered that ethical approval was not required.
Conflict of Interest Statement
All authors declare no competing interests.
Funding Sources
The authors declare they did not receive any funding.
Author Contributions
S.L. drafted the manuscript. J.M.S. and C.L.Z. revised the manuscript. S.L. and J.M.S. searched and selected relevant studies. S.L. and K.Z. extracted the data. K.Z. contributed to the data analysis. All authors read and approved the final manuscript.