Abstract
Background: Mesh-related complications resulting from pelvic organ prolapse (POP) reconstruction operations may be a devastating experience leading to multiple and complex interventions. Objectives: The aim of the study was to describe the experience and time frame of management of mesh-related complications in women treated for POP or stress urinary incontinence in a tertiary center. Methods: 1,530 cases of mesh-related complications were accessed regarding their clinical presentation, number of surgeries, and timeline of surgical treatments to treat multiple clinical complaints until the ultimate operation where all the meshes were removed in a single tertiary center. Results: The studied population revealed to be a highly referred one with only 10.2% of the cases implanted at our center. Clinical presentation varied widely with 48.7% referring pain as the chief complaint, while 31.3% complained of voiding dysfunctions, 2.5% reported genital prolapses, 2.2% complained of vaginal problems, and 1.2% noted intestinal problems as the main clinical complaint. Only 4.8% of the cases presented mesh erosion at examination; 57.8% of the cases required more than 1 operation to address the mesh-related problems. Sixty-eight cases had more than 10 operations up to complete removal. Three clusters of patients could be identified: (i)–those from whom the mesh was promptly removed after clinical problems emerged, (ii) those with slowly evolving problems, and (iii) those with escalating problems despite treatment attempts. Conclusions: Mesh-related complications after pelvic floor reconstruction are an evolving disease with diverse clinical presentation. The identified time-related problems and the multiple failed attempts to treat their complications warrant attention with continuous monitoring of these patients and aggressive removal of the mesh if the clinical complaint cannot be swiftly managed.
Purpose
Pelvic floor reconstruction for pelvic organ prolapse or for the treatment of stress urinary incontinence (SUI) had a dramatic change in the therapeutic approach since the introduction of meshes as an adjunctive tool. Popularization of mesh for pelvic floor reconstruction was rooted in the previous knowledge that many women undergo a repeat operation as a result of recurrence of their condition [1, 2].
Recent evidence has mounted against the initial impression that mesh usage would have no complications. It is clear now that early, late, and very late postoperative complications may occur.
Abundant medical literature for the early postoperative complications (until 30 days postoperatively) was published and did not differ from any other surgical intervention such as bleeding, perforations of internal organs, and hematoma, although mesh implantation was initially conceptualized as minimally invasive, resulting in misreading it as a low-risk operation. Additionally, the unrecognized burden for late or very late complications is a more complex clinical problem due to different bias involved in the identification of the problem.
Attrition bias (lost of follow-up), recall bias (patients with negative results are more prone to report it), and reporting bias (under- or overreporting in injured patients due to stigmatization) are significant forces influencing long-term follow-ups and proper clinical identification by the operating surgeon. Moreover, failed procedures often lead patients to seek other experts, second opinions, and advanced medical resources [3, 4], further confounding the real incidence of mesh-related problems.
These factors were not explored in the context of the mesh-related problems but certainly contribute to the observed concentration of injured patients in tertiary referral centers then well positioned to better understand long-term mesh-related problems. This retrospective cohort study aims to describe an unprecedented amount of patients with mesh-related problems and their surgical timeline to solve their clinical problems since the implantation of the mesh, revealing an astounding number of surgical procedures before complete mesh removal.
Patients and Methods
Institutional Review Board approval was obtained to access the patients’ charts. Patients were identified in retrospect by accessing their electronic files between January 1989 and December 2017. The inclusion criteria were self-referred patients or those referred by a healthcare provider to the Female Pelvic Floor and Reconstructive Medicine Department at the University of California of Los Angeles (UCLA).
Medical history was carefully scrutinized to identify patients’ subjective reason for the visit as well as additional clinical complaints, timeline of the surgeries, and other demographic elements. Details of the mesh-related surgeries were assessed and matched to the clinical history of each case. Eligible cases were all of those with permanent nonabsorbable mesh used to treat pelvic organ prolapse, SUI, or both in one or more pelvic compartments still present at the time of the operation by our department.
We acknowledged that mesh-related complications might be treated elsewhere with different treatment modalities, including outpatient procedures such as office surgical revisions, trimming of exposed meshes, cauterization of the vaginal mucosa, endoscopic resection of the mesh, and others, as well as invasive diagnostic procedures. While some procedures could have had a diagnostic or therapeutic purpose somewhere else, our analysis of the number of previous operations before the definitive removal of all meshes by us considered only patients who were hospitalized to treat mesh-related complications Figure 1.
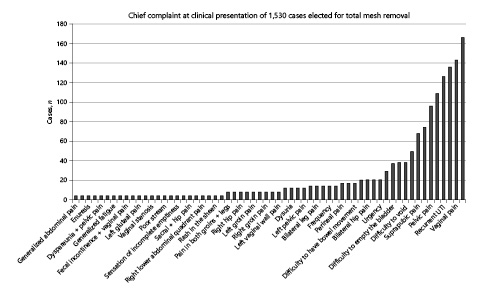
Chief clinical complaint in 1,530 cases of mesh-related problems elected for total mesh removal.
Chief clinical complaint in 1,530 cases of mesh-related problems elected for total mesh removal.
Timeline for each case was established from the point where the mesh was implanted until its complete removal, which determined the final point of our study. The initial point of the timeline was considered the index date where the mesh(es) was(were) implanted with each subsequent operation being carefully identified and graphed with each dot representing a new surgical intervention up to the point of its complete removal at our department.
For the study purpose and graphic visualization, clinical complaints were grouped as initially described by the patient as the reason to seek medical help, despite medical significance or severity of the mesh-related complications found on imaging or physical evaluation. All patients underwent a structured evaluation including history, examination, video-urodynamic study, dynamic MRI of the pelvis, cystoscopy, and translabial ultrasound.
Eight groups were identified in demographics:
retropubic slings and mini-slings were grouped together into the retropubic group;
transobturator slings only;
retropubic slings + anterior vaginal wall repairs with meshes;
transobturator sling + anterior vaginal wall repairs with meshes;
isolated posterior vaginal wall repairs with meshes;
retropubic sling + posterior vaginal wall repairs with meshes;
transobturator sling + posterior vaginal wall repairs with meshes; and
vaginal vault, enterocele, or uterus prolapse repairs with mesh implantation.
For clarity, the patients were divided into arbitrary groups pertaining to the timeline when meshes were completely removed: early removal (1 year after mesh insertion), failed surgery (surgical removal between 1 year and 3 years), late removal (surgical removal between 3 and 10 years), and very late removal (>10 years of insertion of the mesh).
Results
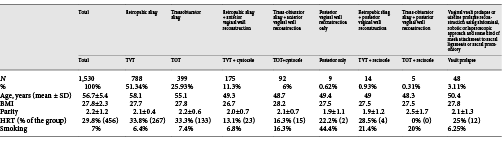
1,530 cases were retrospectively identified and analyzed. The primary surgeon was identified by name in the files of 645 cases (42.1%). One hundred fifty-seven cases (10.2%) had the meshes implanted at the UCLA. Seven hundred twenty-eight women (47.8%) spontaneously sought our department, and in many cases, the primary surgeon could not be identified. Demographics of the studied population are given in Table 1.
As expected, the main complaint from patients opting for mesh removal varied widely, with many exhibiting more than one. Figure 1 depicts the main complaint identified by patients and not by the severity of the problem based on the workup diagnosis. Pain in the abdomen, back, thigh, perineum, suprapubic or bladder region, legs, or vagina was the main reason in 745 cases (48.7%); 479 cases (31.3%) complained of voiding disturbances as the leading cause for their visit; 38 cases (2.5%) reported genital prolapse; 34 subjects (2.2%) reported vagina-related problems such as recurrent vaginal bleeding, chronic vaginal discharge, or vaginal stenosis. Time related to the surgery causing defecation problems or severe constipation was alleged by 18 patients (1.2%). Four patients (0.3%) reported fecal incontinence as their main problem. Systemic symptoms such as fatigue, lupus-like symptoms, arthralgias, and sheen rash were primarily reported by 8 patients (0.5%). Recurrent UTI accounted for 127 cases (8.2%) in the series, and mesh erosion evidenced by the healthcare provider or the patients themselves was described in 74 cases (4.8%).
Figure 2 shows the number of inpatient operations per patient as an attempt to treat the acquired problem after the mesh was implanted until its complete removal. The figure did not include the initial operation where the mesh(es) was(were) implanted but only the number of operations done afterward up to its entire removal.
Number of surgeries each 1 of the 1,530 studied cases underwent before complete mesh removal.
Number of surgeries each 1 of the 1,530 studied cases underwent before complete mesh removal.
In our population, 57.8% of the cases required multiple operations before the mesh was entirely removed. Six hundred forty-six cases had 1 reoperation after the implant of the mesh, meaning that this group of patients had the mesh inserted and completely removed after the detection of some kind of problem – (insertion → complete removal). 347 cases had 1 unsuccessful surgical treatment to correct the problem until the third final and definitive operation of complete mesh removal at our department – (insertion → first unsuccessful attempt → complete removal). 212 patients had 2 unsuccessful surgical attempts before having the mesh completely removed. One hundred eleven cases had 3 unsuccessful surgical attempts, as it follows.
Of note, 27 cases had 10 surgeries, 24 patients had 11 surgeries, and 17 cases had 12 operations done per patient. In total, 68 cases had more than 10 surgeries/patient done after mesh insertion.
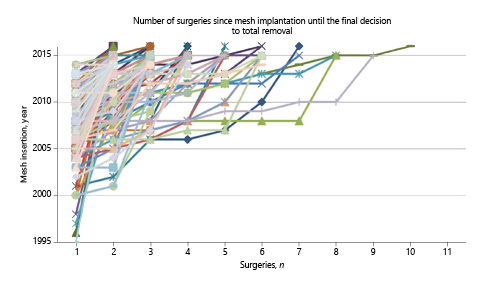
Figure 3 shows the year-related and the sequential number of operations lined for each of the 1,530 studied patients graphically represented. Each dot on the individualized line for each patient in the graph represents an inpatient surgical intervention and its respective year of intervention in the Y axis. It is clear from the graph that many cases had more than 1 operation until the complete removal of the mesh (Fig. 3).
Timeline of sequential therapeutic surgeries in 1,530 cases where problems appeared after mesh(es) were used to reconstruct the pelvis.
Timeline of sequential therapeutic surgeries in 1,530 cases where problems appeared after mesh(es) were used to reconstruct the pelvis.
The steeper the representative case, the fewer the operations were done and longer the time went on until the mesh was removed. More horizontal lines are indicative of shorter period of time to remove the mesh. The longer the line, the higher is the number of surgeries done to treat the complication.
It can also be grossly depicted from Figure 3, 3 clusters of patients who had the mesh removed:
1. Group 1: cluster of cases with a steep inclination. Patients had the mesh removed soon after the mesh-related complications started.
2. Group 2: cluster of cases with oblique inclination (∼45°). Patients had the mesh inserted and clinical problems mounted slowly with persistent unsuccessful surgeries, leading these women to repeated operations throughout an extended period of time.
3. Group 3: cluster with a more flat line. Patients had the mesh inserted and problems emerged or continued in a manner that justified closely repeated surgeries to treat the problem in a short period of time, some of them in the same year.
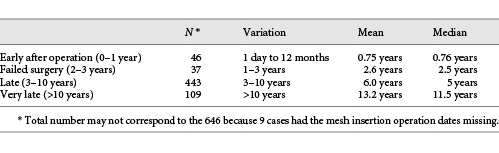
As a whole, group 1 had time elapsed between insertion and removal varying from 1 day to 20 years (mean: 6.5 years; median: 8 years). A closer look at this particular group (group 1 cases [steeper time-line] – 646 cases) (Table 2) – only with insertions and removals of the implanted meshes, 4 patterns of time-elapsed frames could be observed:
646 cases (group 1) classified according to the time ellapsed between insertion and complete removal after detection of mesh-related complications
Group 2 represented 816 cases with more than 2 operations (with a mean of 2.85 operations/patient), along a mean of 7.5 years of assorted attempted operations to solve the clinical problems. Group 3 was represented by 68 cases with >10 surgeries/patient as repetitive attempts to improve the mesh-related problems. This extreme group (group 3) showed an impressive amount of 10.8 operations/patient in a mean time of 7.3-year period anticipating the ultimate decision to completely remove the mesh.
Discussion
The use of meshes to sling urethras or to correct pelvic prolapses represents the most common surgery for SUI [4, 5]. However, until the 2008 FDA public warning, mesh complications were only reported in the medical literature as case reports or small case series studies driving little attention from the medical community [6].
A paucity of the literature reporting on long-term outcomes and complication rates for the use of meshes may have resulted in underreporting of its complications [7]. After the revised work from the FDA on mesh-related complications, it became more evident to the urogynecological community that mesh-related complications were not rare [8]. Cumulative experience on mesh-related complications after prolapse and SUI is now more evident from tertiary referral centers [4, 9, 10], unveiling the burden of suffering and costs for those patients.
We can only be speculative about the reasons why patients develop mesh-related complications. Excessive tensioning of the mesh, urethral obstruction, injury to nerves or tendons by pull-through anchoring devices and erosions are all reasons that can explain the myriad of postoperative symptoms. Likewise, meshes might divert abdominal forces tensioning weakened uterosacral ligaments to result in pelvic and urinary symptoms [11]. The majority of our operated patients were from other centers, and this scenario is not uncommon for other tertiary centers. Unger et al. [2] reported that only 49.3% of their population was managed where the index operation was carried out. This phenomenon was also observed in our case log, with an impressive 89.9% of the cases operated elsewhere.
In particular, our series showed a 58% of our cases requiring multiple operations before reaching our dedicated department. Tijdink et al. [12]. with 36% and others [4, 10] also described this trend in their centers before referral.
Ridgeway et al. [13] reported their experience of mesh removal in 19 cases driven by a composite of pelvic problems. The authors described the patients’ timeline of the problems after mesh implants, and it became clear that mesh complications did not obey any predicted timeline to appear. As in our population, groups of patients of interest could be determined, but for a particular case, time to mesh-related problem could not be predicted [12]. We can only speculate if patients who waited longer to have the mesh removed did so because they coped with the symptoms that posteriorly worsened, if they could not find a professional who linked the problem with the mesh, or if the complaint appeared lately.
Hammett et al. [14], in their short-term surgical outcomes, also reported the unpredictable time to the appearance of problems related to meshes, with 33.7% demonstrating problems within the first 12 months and 64.4% after 1 year. We, as others [4, 15, 16], demonstrated clearly that this population has a high rate of reoperation, with 58% experiencing more than 1 operation. In our view, it seems more appropriate that symptomatic cases should proceed to a formal visit to the operating room in order to remove all meshes ensuring optimal surgical exposure.
Large randomized prospective studies comparing retropubic vs transobturator slings reoperations reported 2.7% of reoperations rate after 12 months [17]. Welk et al. [18] studied 59,887 urethral slings detecting that 2.2% of the cases had the mesh removed after 4.4 years of follow-up. Although large population-based studies report low revision/excision rates for urethral slings–∼ 2–3% [19], a closer look into the data reveals that 64.5% of the cases had a short follow-up in this matter (<2 years) and 86% less than 4 years.
This is in concert with Heinonen’s study who discovered upon an invited visit an unsuspected rate of 32% of mesh erosions after a median 7-year follow-up [20]. Although clinical complaints vary among cases, our patients might represent the more severely affected ones in a dynamic and continuum spectrum of suffering along the timeline. This phenomenon was reported by Funk et al. [7] who showed a low, but regular, incidence of mesh-related problems along the time peaking at 2 years of follow-up. Although these studies provide long-term analysis, it seems from our experience that the appropriate time frame for follow-up is yet to be determined, and probably, these patients should be evaluated periodically in their life span as complications were observed after more than 20 years in some cases.
From that Perspective, iBACKGROUND
Midurethral slings are increasingly used for the treatment of stress incontinence, but there are limited data comparing types of slings and associated complications.
Methods
We performed a multicenter, randomized equivalence trial comparing outcomes with retropubic and transobturator midurethral slings in women with stress incontinence. The primary outcome was treatment success at 12 months according to both objective criteria (a negative stress test, a negative pad test, and no retreatment) and subjective criteria (self-reported absence of symptoms, no leakage episodes recorded, and no retreatment). The predetermined equivalence margin was ±12 percentage points.
Results
A total of 597 women were randomly assigned to a study group; 565 (94.6%) completed the 12-month assessment. The rates of objectively assessed treatment success were 80.8% in the retropubic sling group and 77.7% in the transobturator sling group (3.0 percentage point difference; 95% confidence interval, −3.6 to 9.6). The rates of subjectively assessed success were 62.2 and 55.8%, respectively (6.4 percentage point difference; 95% confidence interval, −1.6 to 14.3). The rates of voiding dysfunction requiring surgery were 2.7% in those who received retropubic slings and 0% in those who received transobturator slings (p = 0.004), and the respective rates of neurologic symptoms were 4.0 and 9.4% (p = 0.01). There were no significant differences between groups in postoperative urge incontinence, satisfaction with the results of the procedure, or quality of life.
Conclusions
The 12-month rates of objectively assessed success of treatment for stress incontinence with the retropubic and transobturator approaches met the prespecified criteria for equivalence; the rates of subjectively assessed success were similar between groups but did not meet the criteria for equivalence. Differences in the complications associated with the 2 procedures should be discussed with patients who are considering surgical treatment for incontinence (ClinicalTrials.gov number, NCT00325039).
It seems clear now that there may be 4 groups of clinical presentation of mesh problems after its insertion:
1. acute urinary retention: it evidently demands immediate attention and may be an early sign of inappropriate technique, excessive tensioning of the mesh, or inappropriate evaluation of the bladder function beforehand;
2. immediate or early pain, bowel dysfunctions, bleeding, or infection but with preserved voiding capacities: it refers to technical problems during insertion or fixation of the mesh causing symptoms that mostly often will demand intervention to correct it;
3. chronic pelvic problems timely related to mesh insertion that aggravate with time in an escalating progress; and
4. very late problems related to the mesh integration to live tissues leading to mesh erosion in hollow organ, fistula, perforations, etc.
The most appropriate approach to these problems has not yet been determined, and referral centers seem to be an opportunity to better understand the best approach and the best time to correct them. Reasons for the mesh-related problems were not clarified, but inappropriate surgical techniques seem to account largely for as commercial kits for vaginal mesh surgery became too popular too rapidly and perhaps not well-trained surgeons lacking appropriate understanding and limitations of the complex pelvic anatomy utilized the techniques.
Moreover, patients treated for pelvic floor problems and SUI seem to constitute a unique population characterized by poor follow-up and unrecognized recurrent rates differing from oncological or transplanted patients [3, 21]. It is becoming common sense that conservative management for mesh-related complications does not provide appropriate management, except in some asymptomatic cases [22].
It is yet to be determined if complete mesh removal is superior to partial removal, but it seems evident from our population that total mesh excision at the first sign of mesh complication seems to be rationally justified as it avoids repetitive and challenged major operations [23, 24].
While it is unclear why some patients moved to another center to solve their problems, it contradicts the perception that the mesh-related complications are minor or easy to solve. We can only speculate for the causes of the mesh complications due to the vast referral pattern observed in our treated population, but surgeons’ expertise seems to adversely affect the outcomes.
Our limitations are the retrospective nature of the study, the heterogeneity of the studied population, and the reasons they presented to the clinic. While it does not solve the problem about the best approach to treat mesh problems, the study clearly demonstrated the necessity for long-term follow-up and the unprecedented burden that some patients face when mesh-related problems emerge after its implantation. Long-term outcome after mesh removal and the subjective clinical presentation are desirable aspects that must be explored in future works on this issue.
Statement of Ethics
All the subjects of this study signed informed consent in accordance with the Helsinki Declaration under the supervision of the Institutional Review Board of the University of California of Los Angeles (UCLA).
Funding Sources
The authors did not receive any funding.
Conflict of Interest Statement
There is no conflict of interest from any of the authors.
Author Contributions
Protocol/project development: Paulo Rodrigues and Shlomo Raz. Data collection or management: Paulo Rodrigues. Data analysis: Paulo Rodrigues and Shlomo Raz. Manuscript writing/editing: Paulo Rodrigues and Shlomo Raz.